Clinical trials for the treatment of coronavirus disease or its symptoms
A snapshot of interventional clinical trials for Covid-19, SARS and MERS in clinicaltrials.gov at the end of April 2020 produces 528 non-vaccine clinical trials. The vast majority (>500) of these are directed at Covid-19. Here, existing therapies are being trialled in one of these Coronaviruses to treat the disease and/or alleviate the symptoms of the disease. A high-level classification of the therapies sheds light on the key therapeutic approaches being explored in these diseases:
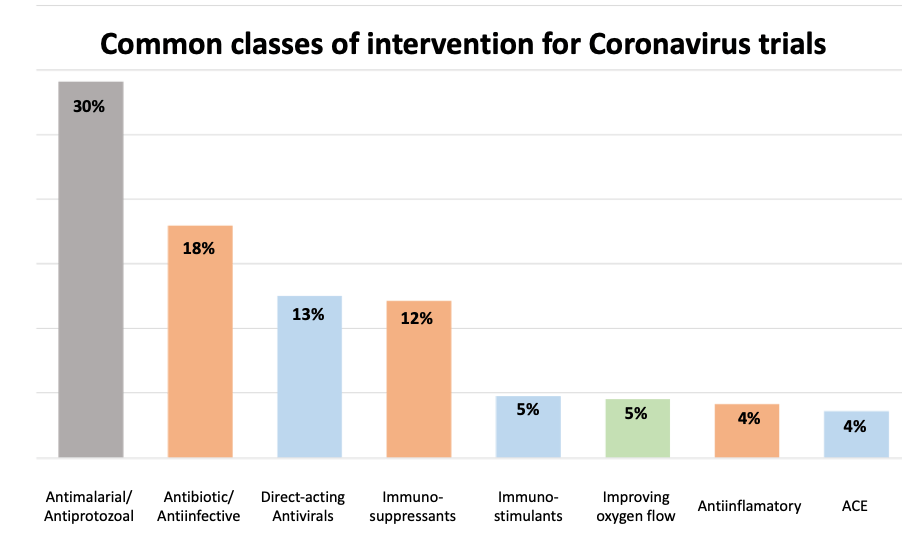
Trials utilising antiviral drugs, either alone or in combination with other mechanisms represent ca. 25% of the current trials (including direct antivirals as well as drugs blocking host interactions such as inhibitors of ACE2). For the live list of drugs being trialled please see our Drug Report.
Trials utilising antiviral drugs, either alone or in combination with other mechanisms represent 30% of the current trials. The drugs currently under investigation are:
Perhaps surprising, 30% of the trials focus on the use of the antimalarial drugs chloroquine and hydroxychloroquine. This is based on reported preclinical ) and early clinical and see commentary in Science Magazine. The efficacy of these drugs in coronaviral infections remains to be validated.
Immunostimulants are being tested alone or in combination in 5% of current studies and typically involve the use of interferons to stimulate immune response as well as the induction of interferon-regulated genes which impact different stages of RNA-viral life-cycle.
Immunosuppressants and anti-inflammatories together comprise 16% of trials and aim to reduce the severe adverse events experienced by patients as a result of elevated Interleukin and other immune/inflammatory responses. Beyond this set, several other trials are testing alternative mechanisms to reduce cytokine response such as the use of the JAK inhibitor, ruxolitinib.
The angiogenic anti-VEGF antibody, bevacizumab is being tested to alleviate pulmonary edema; while 5% of the current trials focus on improving oxygen flow and include the use of Nitrous Oxide and vasodilators.
While most studies have clear rational behind the use of specific therapies, we follow with interest the outcome of the balance between stimulating and suppressing the immune system in this context. For a few trials, however, the strength of the rational remains elusive, such as the use of reverse transcriptase inhibitors.
This analysis represents a static snapshot of clinical trials in April 2020. The landscape is changing significantly. To enable regular review of these trials, we have tagged, where possible, WHO Anatomical Therapeutic Classification (ATC) codes with the trials in our daily-updated clinical trials view of the data.
Trials utilising antiviral drugs, either alone or in combination with other mechanisms represent ca. 25% of the current trials (including direct antivirals as well as drugs blocking host interactions such as inhibitors of ACE2). For the live list of drugs being trialled please see our Drug Report.
Trials utilising antiviral drugs, either alone or in combination with other mechanisms represent 30% of the current trials. The drugs currently under investigation are:
Perhaps surprising, 30% of the trials focus on the use of the antimalarial drugs chloroquine and hydroxychloroquine. This is based on reported preclinical ) and early clinical and see commentary in Science Magazine. The efficacy of these drugs in coronaviral infections remains to be validated.
Immunostimulants are being tested alone or in combination in 5% of current studies and typically involve the use of interferons to stimulate immune response as well as the induction of interferon-regulated genes which impact different stages of RNA-viral life-cycle.
Immunosuppressants and anti-inflammatories together comprise 16% of trials and aim to reduce the severe adverse events experienced by patients as a result of elevated Interleukin and other immune/inflammatory responses. Beyond this set, several other trials are testing alternative mechanisms to reduce cytokine response such as the use of the JAK inhibitor, ruxolitinib.
The angiogenic anti-VEGF antibody, bevacizumab is being tested to alleviate pulmonary edema; while 5% of the current trials focus on improving oxygen flow and include the use of Nitrous Oxide and vasodilators.
While most studies have clear rational behind the use of specific therapies, we follow with interest the outcome of the balance between stimulating and suppressing the immune system in this context. For a few trials, however, the strength of the rational remains elusive, such as the use of reverse transcriptase inhibitors.
This analysis represents a static snapshot of clinical trials in April 2020. The landscape is changing significantly. To enable regular review of these trials, we have tagged, where possible, WHO Anatomical Therapeutic Classification (ATC) codes with the trials in our daily-updated clinical trials view of the data.